


Next: .
Up: Revisiting Woodward Hoffmann Rules
Previous: .
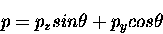 |
(1) |
Since the Hückel scheme is intimately tied to the overlap between orbitals on different carbon atoms,
we next compute the relevant overlap integrals.
Overlap Integrals
The overlap between the orbital on atom 2 and the orbital on atom 1 is
Carl David
1999-06-16