


Next: .
Up: Revisiting Woodward Hoffmann Rules
Previous: The One Electron Hamiltonian
(In the original Woodward and Hoffmann book, the example is
predicated on the transformation of the sp2 terminal hybrids to
sp3 hybrids, so that 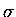 bonds could be formed between atoms
1 and 4. We choose instead to leave the hybridization alone and allow
these terminal carbon atoms to bond using
bonds could be formed between atoms
1 and 4. We choose instead to leave the hybridization alone and allow
these terminal carbon atoms to bond using 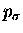 orbitals.
Only the
symmetry considerations matter, so our approach is good enough.
In our simplified approach, we use pz orbitals to form
orbitals.
Only the
symmetry considerations matter, so our approach is good enough.
In our simplified approach, we use pz orbitals to form
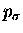 orbitals).
In both cases, the 1-2 and the 3-4 ``
orbitals).
In both cases, the 1-2 and the 3-4 ``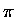 '' bonds are broken, a 2-3 ``
'' bonds are broken, a 2-3 ``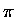 '' bond
forms, and a 1-4 bond forms (
'' bond
forms, and a 1-4 bond forms (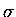 )
(
)
(
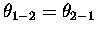 and
and
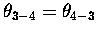 ).
).
Carl David
1999-06-16