| |
(1) |
But we are taught that E=E(S,V), and while it is true (from the Joule expansion
argument), that for an ideal gas, E=E(S) alone, i.e., the energy does not
depend on the volume, never the less, one wonders how to reconcile these two views,
that on the one hand, the energy should be a function of S, and yet it explicitly
is written as a function of T in Equation 1.
How is one to write
Even if one were willing to say that E is a function of T through S, i.e., S= S(T,V) and E=E(S), what are we to do with Equation 1 to construct the Helmholtz free energy (and the Gibbs)?
The Sackur-Tetrode Equation gives us some insight into this problem.
Specifically, this equation is
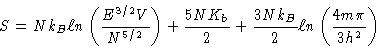 |
(2) |
We can invert Equation 3 solving for E in terms of S and V, obtaining
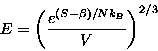
This form begs us to take the partial derivative of E with respect
to S at constant V, to see what happens.
Thus
As an aside, we obtain the other partial derivative
In the set of variables S, T, p, and V the form of the partial derivatives is simplest if one chooses two of them appropriately, the so called canonical variables.
Choosing E(T,V) is not useful, since the partial of E with T at constant V has no special meaning, i.e., does not lead to another member of the set. Choosing E = E(S,V), on the other hand, leads to something wonderful since the partial of E with respect to S leads to T, a member of the set.
The variable pairs S and T on the one hand, and p and V on the other are known as cannonically conjugate variables 3. Posing thermodynamic equations in properly chosen cannonical pairs (one from each pair) results in equations whose information content is maximum, and whose applicable partial derivatives are themselves cannonically conjugate opposites.