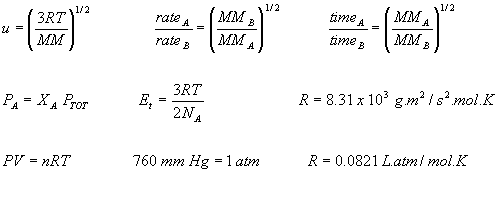
NAME (PRINT) ________________________ SECTION______________
SIGNATURE __________________________ TA ____________________
PLEASE READ THE FOLLOWING INSTRUCTIONS
Do NOT begin the exam until asked to do so.
There are 11 numbered pages, and a periodic table in this exam. Check to see that they are all here before you begin the exam. Return all these papers when you are finished. Write your name on every page. Use a blue or black ink for the entire exam.
Be sure to follow the directions in answering all questions. When solving the problems, SHOW ALL WORK IN A LOGICAL WAY in the space provided. Work done elsewhere will not be considered. Answers that do not show work, no matter how trivial the problem may seem, will receive no partial credit. Place your answers in the blank provided. Use appropriate units and significant figures throughout.
Do not write below this line
Grader
I _____________________ _____________________
II _____________________ _____________________
III _____________________ _____________________
IV _____________________ _____________________
BONUS _____________________ _____________________
TOTAL _____________________ _____________________ ____________
A. (16 points) Answer the following questions on the blanks provided.
___________ 1. What is the oxidation number of hydrogen in KH.
___________ 2. 1.00 L of a solution of Ba(OH)2 contains 0.500 mol of OH-. What is the molarity of Ba(OH)2 in this solution?
___________ 3. Write the formula for the product of the reaction between magnesium nitrate and sodium hydroxide. Answer NR if no reaction occurs.
___________ 4. Of the four acids listed below, which one is a strong acid?
HCℓO, HCℓO2, HCℓO3, HCℓO4
___________ 5. Of the four compounds listed below, which one is NOT a base in aqueous solution?
Ba(OH)2, (CH3)2NH, NO2, NH3
___________ 6. What species are present after the reaction between aqueous ammonia and aqueous HBr?
___________ 7. In a reaction the bromine in HBrO2 becomes Br-. Is bromine oxidized or reduced?
___________ 8. Of the four compounds listed below, which one is insoluble in aqueous solution?
Ni(NO3)3, CrCℓ3, Ca3(PO4)2, MgSO4
B. (10 points) A solution is made up of 35.0 mL of 0.100 M BaBr2 and 45.0 mL of 0.300 M KBr. (Assume there is no reaction between BaBr2 and KBr)
How many moles of bromide ion are in solution?
______________________________
What is the molarity of bromide ion in solution? (Assume the volumes are additive)
______________________________
C. (15 points) In the determination of the molar mass of an unknown acid (HX), a sample of acid weighing 0.1217 g requires 12.37 mL of 0.1164 M NaOH for neutralization to a phenolphthalein endpoint.
How many moles of OH- are used?
______________________________
How many moles of solid acid react?
______________________________
What is the molar mass of the unknown acid?
______________________________
(9 points) 10.0 mL of 0.110 M barium chloride is mixed with 35.0 mL of 0.210 M silver (I) nitrate.
Write a balanced net ionic equation for the reaction. (Pay attention to physical states)
______________________________
How many grams of precipitate are obtained?
______________________________
A. (18 points) Consider the unbalanced equation for a redox reaction in acidic medium:
IO3- (aq) + Mn2+ (aq) → I- (aq) + MnO2 (s)
Write a balanced equation for this reaction using smallest whole number coefficients. The equation itself will not be graded but you will need to balance it to answer the following questions. Write your answers in the blanks provided.
___________ 1. What species is the reducing agent?
___________ 2. How many electrons are gained in the balanced reduction half-equation?
___________ 3. What element is oxidized?
___________ 4. In the balanced redox equation, what is the coefficient for MnO2 (s)?
___________ 5. In the balanced redox equation, what is the sum of the coefficients for the oxidizing agent and H2O?
___________ 6. In the balanced redox equation, is H2O a reactant?
B. (8 points) The active agent in many hair bleaches is hydrogen peroxide. The amount of hydrogen peroxide in a sample of hair bleach can be determined by titration with a standard potassium permanganate (KMnO4) solution. The balanced equation for the titration is:
2MnO4- (aq) + 5H2O2 (aq) + 6H+ (aq) → 5O2 (g) + 2Mn2+ (aq) + 8H2O
If 43.2 mL of 0.105 M KMnO4 is required for this titration, how many moles of H2O2 were present in the sample?
_______________________
How many grams of H2O2 were present in the hair bleach sample?
_______________________
If the mass of the hair bleach sample was 13.8 g, what is the mass percent of H2O2 in the sample?
_______________________
A. (14 points) Answer the questions below, using LT (for is less than), GT (for is greater than), EQ (for is equal to), or MI (for more information required) in the blanks provided.
Consider a steel cylinder (Y) at a given temperature that contains 10 moles of gas A, 10 moles of gas B and 10 moles of gas C.
______ 1. The partial pressure of A (1) the partial pressure of B.
______ 2. In the cylinder, the average kinetic energy of one molecule of A (2) the average kinetic energy of one molecule B.
______ 3. The velocity of one molecule of A (3) the velocity of one molecule of C.
______ 4. If the temperature in the cylinder is increased from 200 K to 400 K, the total pressure in the cylinder at 200 K (4) the total pressure of the cylinder at 400 K.
______ 5. If gas C is replaced without loss by 10.08 g of hydrogen gas, the total pressure in the cylinder before the replacement (5) the total pressure in the cylinder after replacement.
______ 6. The contents of cylinder Y are transferred without loss to cylinder Z which has half the volume of cylinder Y. The temperature remains the same. The total pressure in cylinder Y (6) the total pressure in cylinder Z.
______ 7. Under the conditions of question 6, the partial pressure of gas A (7) the partial pressure of gas B.
B. (5 points) What is the density of oxygen gas at 0.402 atm and 40oC?
_______________________
C. (5 points) A 0.750 g sample of a liquid was vaporized at 125oC. The vapor occupied 330 mL under a pressure of 0.975 atm. What is the molar mass of the liquid?
_______________________
D. (5 points) At 25oC and 380 mm Hg, the rate of effusion of sulfur dioxide through a pinhole is 4.48 mL/s and for an unknown gas it is 6.78 mL/s. What is the molar mass of the unknown gas ?
_______________________
E. (10 points) Consider an experiment where a gas is collected over water. The following data is obtained.
Moles of wet gas = 0.0381 mol
Moles of dry gas = 0.0349 mol
Total pressure = 0.986 atm.
What is the mole fraction of water vapor in wet gas?
_______________________
If the total pressure is 0.986 atm, what is the vapor pressure of water at 42oC?
_______________________
F. (10 points) Nitric acid can be prepared by bubbling dinitrogen pentaoxide into water.
N2O5(g) + H2O→ 2H+ (aq) + 2NO3- (aq)
450 mL of a 0.200 M solution of H+ were produced. What is the number of moles of H+ produced?
_______________________
What volume of dinitrogen pentaoxide at 25oC and 0.980 atm is required to produce 450 mL of 0.200 M H+? (see the solution mentioned in question 1)
_______________________
A. (5 points) A mixture of NH4Cℓ and NaHCO3 is to be separated by fractional crystallization. The following data is given:
Mass of NaHCO3 in mixture 25. 0 g
Mass of NH4Cℓ in mixture 8.0 g
Temperature of mixture 50 oC
solubility of the NH4Cℓ at 50oC 24.0 g NH4Cℓ/100 g water
solubility of NaHCO3 12.0 g NaHCO3/100 g water
Assume that the solubility of one substance is not affected by the presence of another. Calculate the minimum amount of water required to dissolve the mixture.
________________________
B. (20 points) In order to determine the chemical formula of a compound containing chromium and oxygen, an analysis is carried out that starts with 5.9962 g of CrxOy. The mass of chromium metal obtained is 4.1026 g.
How many moles of Cr are in the sample?
________________________
How many grams of O are in the sample?
________________________
How many moles of O are in the sample?
________________________
What is the mole ratio of O:Cr in the sample?
________________________
What is the formula of the chromium oxide?
________________________
BONUS (15 points) ALL or NOTHING
The bonus should be done only after you have completed the main part of this exam and checked your work for errors. The time allotted for this exam does not include time for the bonus.
One method to coat a copper item with a layer of silver relies on the following reaction: Copper metal, when brought into contact with a solution containing silver (I) ions, reduces the silver (I) ions to metallic silver. As the silver is deposited, the copper is oxidized to copper (II) ions which go into solution.
A copper strip with a mass of 2.00 g is dipped into a solution of AgNO3. After some time has elapsed, the strip is removed from solution and is dried. It was found to weigh 4.18 g. What are the masses of copper and silver in the strip ? (YOU HAVE TO GIVE THE MASSES OF BOTH SILVER AND COPPER)
_____________________ Ag _____________________ Cu