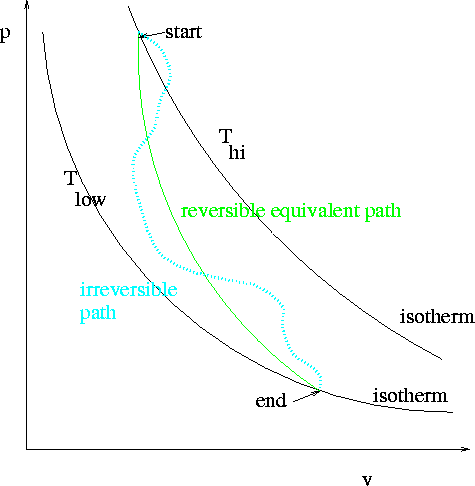
Consider a mole of ideal gas at Tstart , pstart, and Vstart. We keep the gas in a traditional piston cylinder arrangement. Now we place stops (chocks) on the piston to prevent its upward motion and then we reduce the pressure on the outside to pend. The chocks provide the (extra) equivalent force so that initially, the gas is still being subject to a pressure of pstart. Next, we plunge the piston cylinder apparatus into a large temperature bath (standard disclaimers apply), whose temperature will remain constant at Tlow, while simultaneously removing the chocks, so that the gas suddenly "sees" only pend.
There is no question about what will happen next. The gas will expand and cool, irreversibly. It will do so spontaneously. Tlow is lower than Thi (by definition).
We are conducting an irreversible expansion of an ideal gas against a constant (lower pressure) while simultaneously cooling it. At the end of the expansion/cooling, Tlow, since we used a large heat bath, pend, i.e., the chocks having been removed, the only pressure is the final one, and the final volume is computable from the ideal gas law.
If we were smart enough to set the conditions so
that the final equilibrium position of the piston, once attained, corresponded
to increasing the volume to a value which places us where:
 |
It is hard to imagine that the process illustrated could,
spontaneously and by its own devising, proceed backwards from our final to our
initial state.
Thus we have associated a decrease in energy at constant entropy with
spontaneity in the same way we normally associate an increase in entropy at
constant energy with that selfsame spontaneity.
That function which combines both the entropy increase and the energy
decrease associated with spontaneity (A)
has been chosen (E-TS) so that if either E decreases or S increases
(with the other constant)
the function drops in value.
Further, this function shows us that entropy increases with contravening
energy increases can lead to non-spontaneous processes, and entropy
decreases coupled with energy decreases can also
lead to spontaneity.