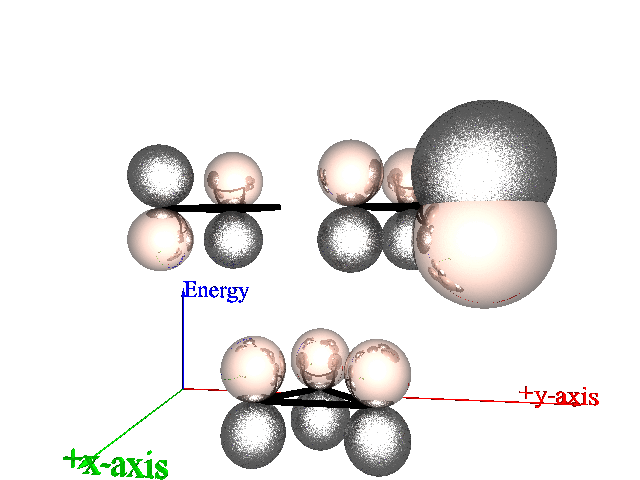
Here are the orbitals of cyclopropene, two degnerate anti-bonding orbitals,
and one bonding orbital, according to elementary Huckel Theory.
 Spherical Harmonics (s thru f orbitals)
Spherical Harmonics (s thru f orbitals)
Radial Hydrogenlike Wave Functions
| To-> | e.V. | kcal/mol | cm-1 |
sec-1 | erg |
| From e.V. | 1 | 23.05 | 8.065 x 103 | 2.43 x 1014 | 1.601 x 10-12 |
| From kcal/mol | 4.345 x 10-2 | 1 | 350 |
1.05 x 1013 | 6.95 x 10-14 |
| From cm-1 | 1.24 x 10-4 |
2.858 x 10-3 | 1 | 3 x 1010(c) | 1.986 x 10-16(hc) |
| From sec-1 | 4.13 x 10-15 | 9.52 x 10-14 |
3.33 x 10-11 | 1 | 6.627 x 10-27(h) |
| From erg | 6.25 x 1011 | 1.439 x 1013 | 5.04 x 1015 |
1.51 x 1026 | 1 |
e = 4.80286 x 10-10 esu
me = 9.1055 x 10-28gram
mproton = 1.67312 x 10-24 gram
h = 6.627 x 10-27erg-sec
aBohr = 0.5296 x 10-8cm
kBoltzmann = 1.38033 x 10-16ergs/K
c = 299793.3 km/sec
Questions
-
Trial Wavefunction for H2+ Cation
-
Trial Wavefunction for H2+ Cation (II)
-
Trial Wavefunction for H2+ Cation (III)
-
Hamiltonian on Trial Wavefunction for H2+ Cation
-
Overlap Integration on Trial Wavefunction for H2+ Cation
-
Overlap Integral on Part of Trial Wavefunction for H2+ Cation
-
Molecular Orbital Filling (Aufbau Prinzip)
-
Where Are Electrons in MO's
-
Correlations using LCAO's
-
Appropriate AO's in Water's MO's
-
Appropriate sp3 Orbital's in Water's MO's
-
Probability of Finding Electron Below x-y plane in HeH+
 Spherical Harmonics (s thru f orbitals)
Spherical Harmonics (s thru f orbitals)